Richard N. Upton
Department of Anaesthesia and Intensive Care, Royal Adelaide Hospital and University of Adelaide, North Terrace, Adelaide, SA 5005, Australia.
The development and practice of intravenous anaesthesia has advanced pharmacokinetic knowledge more than any other form of pharmacological therapeutics. The 2 or 3 compartment mamillary model has been at centre of these advances, and has the honour of being the only pharmacokinetic model to have a direct clinical application via target controlled infusions. Population pharmacokinetic approaches using this type of model have proven useful in quantifying inter and intra-patient variability in the kinetics and dynamics of intravenous anaesthetics and analgesics. However, this variability is relatively high. The remaining challenge in this area is to understand the mechanistic basis of this variability. To do so may mean looking beyond our trusted servant - the mamillary compartmental model.
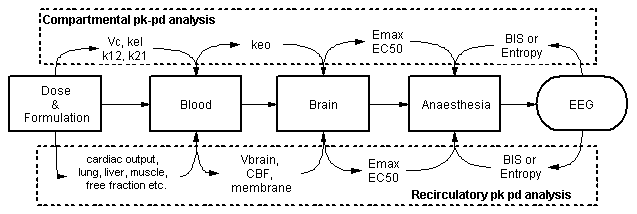
It is known from first principles that the flow of blood transports drugs around the body - but there are no parameters for blood flow(s) in a mamillary compartmental model. If a hypothesis is proposed that variability in blood flow is one of the factors underlying variability in the kinetics of intravenous anaesthetics, then new models incorporating blood flows as parameters are needed. Full physiological models are an option, but not all organs or blood flows are of interest. An emerging alternative is recirculatory pharmacokinetic models, which in general have cardiac output as a parameter, a representation of the lungs and a lumped description of the remainder of the body [1-6]. They are particularly suited to describing i.v. bolus or "front end" kinetics.
In mamillary compartmental models, drug effects are related to blood concentrations via a hypothetical effect compartment with a rate constant keo. What is the equivalent of an effect compartment in recirculatory modelling? While it is quite feasible to use an empirical effect compartment, it is not in the spirit of physiological fidelity. Fortunately, for many drugs, it is possible to identify a specific organ where the drug exerts its therapeutic effect. For intravenous anaesthetics, it is quite clear that this organ should be the brain:

Studies in animals and man have shown that the depth of anaesthesia is directly related to the global concentration of anaesthetic in the brain [6]. The effect compartment rate constant can therefore be recast as the factors that affect the rate of equilibration of the brain and blood drug concentrations. These include 1) the volume of distribution of the drug in the brain, 2) cerebral blood flow, 3) BBB permeability and 4) the presence or absence of active BBB transport. For lipophilic intravenous anaesthetics, usually it is only the first two factors that are of importance. High cerebral distribution volumes and low cerebral blood flow states delay the equilibration between the blood and the brain, and vice versa. Changes in free fraction in the blood affect cerebral distribution volume.
A single time-course of arterial blood concentrations in a patient does not contain all the information needed to parameterise a recirculatory pharmacokinetic with the brain as a target organ. At the least, some information is required about cardiac output and cerebral blood flow. We have some understanding of the mean values of these flows in the population from the literature. In individual patients, non-invasive methods of measuring these flows are developing rapidly. The modelling process is also greatly facilitated by data on drug kinetics in individual organs. In man, these data can be collected via regional blood sampling. Thus, jugular bulb blood sampling provides information about cerebral kinetics [7]. Pulmonary artery blood sampling provides information about lung kinetics [8], and renal vein sampling provides information about renal kinetics [8]. Small opportunistic studies of this type in surgical patients can provide a wealth of data.
Analysis of recirculatory pharmacokinetic models with a brain target shows that the time-course of the brain concentrations (and therefore anaesthetic effects) are influenced by cardiac output and cerebral blood flow for bolus doses, and by cardiac output at steady state. High cardiac outputs will produce lower steady state concentrations by two mechanisms - a direct dilution effect and by increasing hepatic clearance. The arterial blood concentrations of intravenous anaesthetics can respond quickly to changes in cardiac output. For example, animal studies have shown that infusing catecholamines for 15 min during steady state anaesthesia with propofol could raise cardiac output. The increase was sufficient that the blood and brain concentrations of propofol became low enough for the animals to emerge from anaesthesia [9].
What then are the factors that can affect these important blood flows in surgical patients? Cerebral blood flow is affected amongst others by the metabolic rate of the brain, carbon dioxide tension and the presence of drugs with cerebral vasoconstrictor or dilator properties. It is important to remember that intravenous anaesthetics reduce cerebral blood flow by up to 50%, and therefore affect their own kinetics in the brain. Cardiac output is affected amongst others by the state of hydration and blood volume, haematocrit, carbon dioxide tension and the state of sympathetic stimulation. Cardiac output drops by nearly 50% with increasing age. The current haemodynamic status of a patient can be thought of as the result of a two-stage process - first the basal haemodynamic status determined by allometric considerations - age, weight and sex which could be predicted to some extent a priori. Second is any change in this basal state caused by disease, other drugs and acute physiological and pathophysiological events. This is more ephemeral, and may change during the course of surgery. It can be speculated that a stable intravenous anaesthetic will depend on maintaining a stable haemodynamic status. In particular, transient rises in cardiac output due to acute sympathetic stimulation should be avoided.
The recirculatory model with a brain target certainly has a role in elucidating basic mechanisms underlying variability in the disposition and effects of intravenous anaesthetics. It has the potential to increase the fidelity of patient simulators in the training of anaesthetists. A direct clinical application may become possible with advances in non-invasive blood flow measurement, but will require careful validation and a demonstrated advantage.
1. Weiss M, Forster W. Pharmacokinetic model based on circulatory transport. Eur J Clin Pharmacol 1979; 16:287-93.
2. Avram MJ, Krejcie TC. Using front-end kinetics to optimize target-controlled drug infusions. Anesthesiology 2003; 99(5):1078-86.
3. Cutler DJ. A linear recirculation model for drug disposition. J Pharmacokinet Biopharm 1979; 7:101-16.
4. Lam G, Chen ML, Chiou WL. Determination of tissue to blood partition coefficients in physiologically-based pharmacokinetic studies. J Pharm Sci 1982; 71:454-6.
5. Kuipers JA, Boer F, Olofsen E, Olieman W, Vletter AA, Burm AG, Bovill JG. Recirculatory and compartmental pharmacokinetic modeling of alfentanil in pigs: the influence of cardiac output. Anesthesiology 1999; 90(4):1146-57.
6. Upton RN, Ludbrook GL. A physiologically based, recirculatory model of the kinetics and dynamics of propofol in man. Anesthesiology 2005; 103(2):344-52.
7. Ludbrook GL, Visco E, Lam AM. Propofol : Relation between brain concentrations, electroencephalogram, middle cerebral artery blood flow velocity, and cerebral oxygen extraction during induction of anesthesia. Anesthesiology 2002; 97:1363-70.
8. Hiraoka H, Yamamoto K, Miyoshi S, Morita T, Nakamura K, Kadoi Y, Kunimoto F, Horiuchi R. Kidneys contribute to the extrahepatic clearance of propofol in humans, but not lungs and brain. Br J Clin Pharmacol 2005; 60(2):176-82.
9. Myburgh JA, Upton RN, Grant C, Martinez A. Epinephrine, norepinephrine and dopamine infusions decrease propofol concentrations during continuous propofol infusion in an ovine model. Intensive Care Med 2001; 27(1):276-82